2015 Spring
Materials for energy and environment
A1Carbon dioxide recovery and circular economy of carbon
Carbon dioxide is one of the essential molecules for life cycle and energy. Its storage has a key role for human activities and can be summarized as the starting step for biomass production but also the final step of combustion processes. These two key steps identify the storage of all human and biosphere activities. Today the question is how to control the large amount of carbon dioxide emission which is responsible of climate change but at the same time, the energy needed for 7 billion of capita cannot be neglected.
Today, carbon dioxide can be used through a new circular economy by industrial processes, opening the way of both energy storage and network regulation. However, to reach these objectives, new electrotechnical processes, photocatalysis and photolysis of water have to be developed, in order also to create new polymers.
Scope of this two days symposium (combined with symposium A: Materials, mechanisms and devices in nano energy) is to discuss the current R&D situation, especially by considering the materials aspects in our E4F model for a future sustainable energy supply for the whole world.
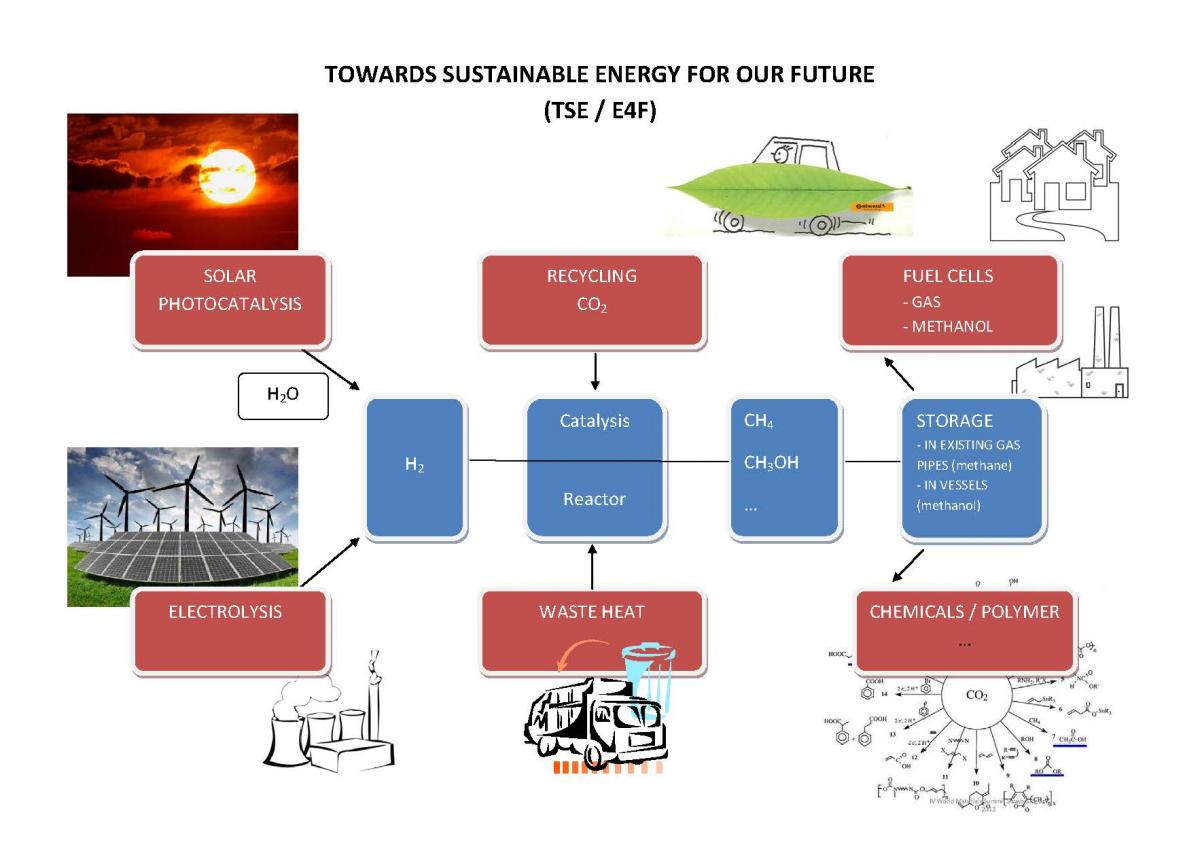
Both fundamental and technology oriented researches in materials being mandatory to contribute to solve the problem related to the need of reduction of CO2 in the atmosphere and to store the electricity generated by renewable energy sources, contributions are sollicitated in the following areas:
- CO2 sources and purification
- Splitting and recovery of CO2 by innovative technologies, especially catalysts and
- plasma processes
- Generation of new fields including solvent properties, dyeing, EOR, energy storage and synfuels
- Organic chemistry processes based on CO2 recoverymainly for biodegradable-polymers
Tentative list of scientific committee members :
- S. Biollaz, PSI, Switzerland
- K. Hashimoto, Tohoku University, Institute for Materials Research, Japan
- O. Kröcher, Bioenergy and Catalysis Laboratory, PSI, Switzerland
- J. R. Morante, IREC, Catalonia Institute for Energy Research, Spain
- P. Rutberg, Institute for Electrophysics and Electric Power, Polytechnical State University, Saint Petersburg, Russia
- C. Sanchez, Collège de France, Paris
Scientific Committee:
- L. Bedel (CEA & CEOPS consortium)
- T. Lippert (E-MRS / PSI)
- R. Martins (University of Lisbon)
- J. P. Massué (E-MRS)
- G. Mignani (Solvay)
- P. Siffert (E-MRS)
Supported by:
 |
 |
This symposium is organized in the frame of the CEOPS project, which received funding from the European Union Seventh Framework Programme under grant agreement No 309984.
| Start at | Subject View All | Num. | |
|---|---|---|---|
General introduction : R. Martins | |||
| 09:00 | Authors : R. Martins Affiliations : E-MRS Resume : ... | A1.A1.1 | |
Point of view of a pioneer : - | |||
| 09:15 | Authors : Koji Hashimoto1, Naokazu Kumagai2, Koichi Izumiya2, Hiroyuki Takano2 and Zenta Kato1 Affiliations : 1Tohoku Institute of Technology, Sendai 982-8577 Japan 2Hitachi Zosen Corporation, Kashiwa 277-8515 Japan Resume : The increase in world energy consumption at the current rate will lead to complete exhaustion of world reserves of oil, natural gas, uranium and coal until the middle of this century. In order to avoid the crisis of no fuels and intolerable global warming, we need to establish the technology by which whole world can survive using only renewable energy. We have been performing research and development for the supply of renewable energy in the form of methane via electrolytic hydrogen generation using carbon dioxide as a feedstock since 25 years ago. We created very effective catalysts for carbon dioxide methantaion, and anode and cathode for hydrogen production by seawater electrolysis. We constructed prototype plant in 1995 consisting of PV, seawater electrolyzer for hydrogen production, carbon dioxide methanation plant and methane combustor from which carbon dioxide was sent back to the methanation plant. In 2003 we built pilot plants of industrial scale consisting of seawater electrolyzer and carbon dioxide methanation plant of 1 Nm3/h methane production rate. Since the activity of our oxygen evolution anode for direct seawater electrolysis is not sufficient, for immediate actual industrialization we have been creating energy-saving anode and cathode for hydrogen production by alkaline water electrolysis. Since 2012 an international joint R & D is in progress for conversion of carbon dioxide in crude natural gas to methane combined with production of hydrogen by alkali water electrolysis using intermittent electricity generated by renewable energy. | A1.A2.1 | |
| 11:00 | Authors : Gérard Mignani Affiliations : Solvay Resume : CO2 is an important building block for the industry: the markets are profitable and substantial. This starting buildingblock can be used in the production of composite materials based on polycarbonates, as energy vector (Methanol, formic acid), as an intermediary in many chemical industry areas. The use of CO2 can be direct or indirect such in recovery of oil domain, supercritical CO2 is currently becoming very important for extraction and purification industrial processes. From the industrial perspective, CO2 has the advantages of being available industrially in very large quantities, renewable and non-toxic but with very variable chemical purities. For organic synthesis,it can replace toxic products such as phosgene or homologues. Lastly, it provides access to existing products, but also more interesting new ones. One of the real challengesis to combine efficient processes, cost effectiveness and environmental exigencies with low footprint. New catalytic materials are needed to transform industrial CO2 flew into desired derivatives or materials. The use of CO2 in polycarbonates has been particularly well studded and developed. Using specific catalysts, CO2 can be used as an oxidant for certainindustrial processes reactions. The CO2-hydrates can be an interesting raw material for relevant chemical and physical applications. Methanol is a very important chemical intermediate for synthesis of various chemicals derivatives such as acetic acid and dimethyl ether that is the key intermediate for olefins (MTO) and gasoline (MTG) products. The VITESSE2 project of ANR French National Agency has contributed to develop a new industrial approach to the use of CO2 and hydrogen produced by water electrolysis using surplus of electricity from decarbonized sources. In conclusion, all the studies carried out point clearly to CO2 becoming an essential building block for the chemical industry. However, to make this an industrial reality, close collaboration between academia and industry is necessary. Strategically and industrially the re-use of CO2 is a sustainable development challenge for our economies and strongly connected with the clean coal development. | A1.A4.2 | |
| 12:00 | Authors : Stephan Rieke Affiliations : Etogas GmbH Resume : ... | A1.A4.4 | |
| 12:30 | Authors : I. Kumkova & V. Popov Affiliations : RAS Resume : ... | A1.A4.5 | |
The European project CEOPS & its results : - | |||
| 14:30 | Authors : L. Bedel & consortium members Affiliations : CEA Grenoble - LITEN-DTBH - 38054 Grenoble Cedex 9 - France Resume : CEOPS is a NMP-FP7 European project including 10 partners. Itproposes a sustainable approach for the production of methanol from carbon dioxide. CEOPS concept is based on a use of the existing wide natural gas network via the injection and transportation of an intermediate product between carbon dioxide and methanol: methane.The concept of the project relies on the development of two chemical pathways through electro-catalytic processes: (i) carbon dioxideconversionto methane and (ii) the direct conversion of methane to methanol.The results have demonstrated significant progresses beyond the state of the art in both pathways and a prototype system is under construction and it will be tested by the end of the project. A techno-economic and environmental studies will assess the performance gain of the technological rupture proposed by the project. | A1.A5.1 | |
| 14:50 | Authors : A. Stephane, M.Jouve, P.Baurens, J.Amouroux, S.Cavadias, M.Nizio, J.-R.Morante, T.Andreu, C.Henriques, I.Graça, A.Neto. Affiliations : CEA Grenoble - LITEN - 38054 Grenoble cedex 9 Resume : The synergetic effect of catalyst andsurface non-thermal dielectric barrier discharge plasma on carbon dioxide reduction into methanewas investigated in a fluidized bed reactor packed with Ni/-Al2O3 catalyst particles (200-300µm).No CO2conversion was observed with only plasmaignitionbut high CO2conversion wasobtainedby thermal catalysis alone (>65%) in the temperature range of 250-330°C and the pressure range of 3 to 5.5bar. Reaction products, including CH4and some amounts of CO, were identified by using a µGC.Higher CO2conversion efficiencies were achievedwith plasma addition thanks to catalyst activity promotion, especially at higher injected power where results showed up to +6% in the CO2 conversion rate for an energy consumption of about 50kJ/mole of produced CH4.An increase of the injected power and of the temperature resulted ina slight diminutionof CH4 selectivity but remaining higher than 97% in all performed experiments. | A1.A5.2 | |
| 15:10 | Authors : J.Amouroux, S.Cavadias, M.Nizio and S. Ognier Affiliations : Equipe 2PM - ENSCP - IRCP, UMR 8247, 11 rue P. et M. Curie 75005 Paris Université P. et M. Curie, 4 place Jussieu, 75005 Paris Resume : In the context of reducing emission of greenhouse gases to the atmosphere the direct conversion of carbon dioxide and methane to methanol has become an important research topic. Encouraging results on the hydrogenation of carbon dioxide in a dielectric - barrier discharge (silent discharge) reactor with the aid of a catalyst in the plasma zone have already been obtained. The aim is to transform CO2 - CH4 using the Sabatier reaction: CO2 + 4H2=> CH4 +2H2O ΔH = - 165KJ/mol at low temperature by using a catalyst activated by a DBD plasma. The exothermicity of the reaction leads to an increase of temperature. Above 400°C and CO + H2 are major products. In order to keep temperatures in the range of Sabatier reaction (350°C-400°C) and avoid the ageing of the catalysis the reactor must be cooled. The key step of the reaction, at this range of temperature is the water desorption from the catalytic sites. The catalyst in the tubular reactor activated by the high frequency streamers of electrons leading to its polarization, that controls kinetic mechanisms of adsorption and desorption all the species (CO2, H2, CH4 and H2O). This exothermic reaction is performed on catalysts with specific characteristics in terms of thermal and electrical conductivity, activated by high voltage (~10kV) DC, pulsed discharges (ns to µs) leading to the polarization of the bulk in order to increase the kinetic rate of the reaction. It enhances the desorption of water and control the thermal effect. The adsorption and desorption rate are controlled by the polarization of the Homo and Lumo orbitals of the catalytic sites during the elementary pulses, that is why an increase of the desorption rate of H2O leads to an increase of the global conversion rate of CO2 at low temperature (100°C) and an increase simultaneously of the time life of the catalyst (no deposition). The action of the plasma is an increasing of the conversion rate of CO2 to CH4 in all cases, using these specific catalysts, for a range of temperature between 320 to 420 °C. Moreover at low temperature range, 130°C-150°C, the conversion jumps from 1% (catalyst without discharge) to 78 %-85% (catalyst + discharge) and the selectivity to CH4 formation is near to 100%. Our results point out that catalyst and plasma open the way of a new process with large energy efficiency, large conversion rate up to 90% and a time life of the catalyst higher because it works at low temperature. | A1.A5.3 | |
| 15:30 | Authors : Teresa Andreu, Sebastián Murcia-López, Katherine Villa, Joan R. Morante Affiliations : IREC, CatalonianInstituteforEnergy Research, Jardíns de les Dones de Negre,1. San Adrià del Besòs. 08930. Spain. Resume : The introduction of renewal sources of energy has accelerated the interest in the power to gas concept, where renewable hydrogencan be used to synthesize synthetic natural gas (SNG) by hydrogenation of carbon dioxide via Sabatier reaction. SNG, as energy carrier, can be then stored and distributed safely in huge quantities in the existing natural gas infrastructure. Methane, as major component of natural gas, can be later transformed into oxygenated and longer chain products for its use as fuel or fine chemical, such as methanol. In this context, the use of light and water to convert methane into methanol is an attractive option, using a photocatalytic approach. In this work, recent results on the use of mesoporousWO3, Bi2WO6 and BiVO4 as photocatalyst will be presented, as well as the use of homogeneous additives, either hydroxyl radical (OH) or electron scavengers. We will present how an intimate synergy between the semiconductor photocatalyst features (electronic and surface properties, oxidizing potential) and media conditions must be considered in order to optimize the selectivity and yield of the photocatalytic reaction. | A1.A5.4 | |
| 15:50 | Authors : Noémie Elgrishi,[a] Matthew B. Chambers,[a] Xia Wang,[a] Christopher H. Hendon,[b] Aron Walsh,[b] Jonathan Bonnefoy,[c] Jérôme Canivet,[c] Elsje Alessandra Quadrelli,[d] David Farrusseng,[c] Caroline Mellot-Draznieks[a] and Marc Fontecave[a] Affiliations : a) Collège de France, "Chaire de Chimie des Processus Biologiques" b) Department of Chemistry, University of Bath, UK c) IRCELYON, Université Lyon 1 CNRS, UMR 5256, France d) C2P2, Université Lyon 1 CPE CNRS, UMR 5265, France Resume : The confinement of organometallic catalyst inside the cavity of porous materials was reported to enhance selectivity and activity.[1,2] Metal-Organic-Framework appear to be appealing platforms for immobilization of organometallic catalyst as already reported by our group.[3] The first photosensitization of a rhodium-based catalytic system for CO2 reduction is reported, with formate as the sole carbon-containing product.[4] Heterogenization of molecular catalysts via the synthesis of a new metal-organic framework (MOF) Cp*Rh@UiO-67 obtained through post-synthetic ligand exchange. While the catalytic activities of the homogeneous and heterogeneous systems are found to be comparable, the MOF-based system is more stable and selective. Furthermore it can be recycled without loss of activity. For formate production, an optimal catalyst loading of ~10% molar Rh incorporation is determined. Through the study of the behaviour of MOF systems having a controlled Rh loading, competitive catalytic reaction occurring inside the Cp*Rh@UiO-67 framework are postulated. [1] J.M. Thomas et al ,Acc.Chem.Res, 2008, 41, 708-720. [2] A. Corma et al. , J.Chem.Commun,Chem.Commun. 1991, 1253-1255. [3] J. Canivet, S. Aguado, Y. Schuurman, D. Farrusseng, J. Am. Chem. Soc. 2013, 135, 4195−4198 [4] M. B. Chambers, X. Wang, N. Elgrishi, C. H. Hendon, A. Walsh, J. Bonnefoy, J. Canivet, E. A. Quadrelli, D. Farrusseng, C. Mellot-Draznieks, M. Fontecave, ChemSusChem 2015, DOI: 10.1002/cssc.201403345. | A1.A5.5 | |
| 16:40 | Authors : S. Messias(1), J. Afonso(1), A. S. R. Machado(1) *, T. R. C. Fernandes(1), T. Pardal(1),C.M. Rangel(2), D. Nunes(3), R. Martins(3), Zeljko Petrovsky(3) and Manuel Nunes-da-Ponte(3) Affiliations : (1) Omnidea, Lda., Travessa António Gedeão. No. 9, 3510-017 Viseu, Portugal (2) Laboratório Nacional de Energia e Geologia, Estrada do Paço do Lumiar, 22, 1649-038 Lisboa, Portugal, (3) Faculdade de Ciências e Tecnologia da Universidade Nova de Lisboa, 2829-519 Caparica, Portugal *ana.machado@omnidea.net Resume : Electrochemical-assisted CO2 reduction is a technology that can represent a contribution to the reduction of the emission of greenhouse gases by using CO2 as a raw material for fuel production, or for value added chemicals [1]. However, major technological challenges have prevented this technology to reach industrial maturityup to now. This work discusses the challenges presented by this technology. Improvements beyond the state-of-the art are reported. Copper based catalytic cathodes performance is described in terms of faradaic efficiencies, reversibility and product selectivity. Characterization of these materials by cyclic voltammetry and scanning electrode microscopy are presented. Recently task-specific ionic liquids with high solvent power towards carbon dioxide are being developedbeing significantly better solvents for CO2 thanwater [2]. The couple catalytic cathode/ionic liquid based electrolyte plays a determinant role in the performance of the electrochemical system. The influence in the electrochemical reaction of CO2 reduction of parameterssuch as voltage applied, pressure, electrolyte composition are reported. KEYWORDS: Carbon dioxide;ionic liquids; electrochemical reduction; catalysis. REFERENCES [1]G. Centi, S. Perathoner,Catalysis Today 148 (2009) 191205 [2] J. E. Brennecke, B. E. Gurkan, JPC Lett. 1 (2010) 3459-3464. | A1.A5.6 | |
| 17:00 | Authors : Xianhong Wang Affiliations : Key lab of Polymer Ecomaterials of Sciences, Changchun Institute of Applied Chemistry, CAS Resume : ... | A1.5.8 | |
European initiatives : - | |||
| 17:50 | Authors : Silvia Gross Affiliations : IENI-CNR Resume : ... | A1.A6.1 | |
Link to symposium A : - | |||
| 18:20 | Authors : J.R.Morante(1),(2) Affiliations : (1) IREC, Catalonian Institute for Energy Research, Jardíns de les Dones de Negre,1. San Adrià del Besòs 08930. Spain. and Faculty of Physics, University of Barcelona. C/ Martí i Franquès 1, Barcelona 08028 Spain. Resume : Solar energy can be used as source to have with thermal or electrical energy or alternatively solar photons can also be used for direct conversion of solar energy to the chemical one. Moreover, assuming that CO2 and H2O feed-stocks are adequately available, there are all the conditions for producing solar fuels from the photoelectrochemical processes working like a solar refinery. The main products of this refinery are hydrogen from water splitting process, synthetic methane from CO2 + solar H2 or other added value chemical from the direct reduction of CO2 such as methanol, formic acid, syngas, etc. Nowadays, the expected efficiencies for the direct solar to hydrogen conversion are higher than the found values for applying photovoltaic plus one alkaline electrolysis system. Here we provide a concise review on the key issues about PEC processes and involved materials and configurations in order to scale up PEC systems for a high throughput production. Likewise, our focus is on the energy balance among the used energy from the solar spectra, the electrical power consumption and the produced hydrogen or C1 molecules. Features like photocurrent density and required bias or productivity and selectivity will be considered, analyzed and discussed. Special attention will also be paid to the combination of photo electrode materials and electrocatalyst layers. A first estimation of production costs will also be advanced. | A1.A7.1 | |
| 18:40 | Authors : Michael Anthony Gleeson, Tesfaye Belete, Anton Walsh, Shaoying Wang, Richard van de Sanden Affiliations : Dutch Institute for Fundamental Energy Research (DIFFER) Resume : The combination of (catalytically-active) materials and plasma-processing has the potential to offer new possibilities for the efficient conversion of CO2 to either CO or hydrocarbons using electrical energy. Plasma offers a route for the direct conversion of electrical to chemical energy, while materials can provide the necessary product selectivity and conversion efficiency. Plasma-catalytic synergies are commonly observed. In such a reactor scheme, the solid surfaces are constantly in a processing environment during operation under continuous exposure to photons and bombardment by charged and excited species. To be successful, this approach requires fundamental understanding of the details of the plasma-surface interaction and the development of materials that are specifically tailored for maximum utilization of the plasma environment. We will provide a status report on current efforts at the Dutch Institute for Fundamental Energy Research (DIFFER) to evaluate and utilize the potential for material-mediated plasma-assisted conversion of CO2. This will cover three specific topics: investigations on fundamentals aspect of the interactions of plasma-excited CO2 at surfaces; work on tailoring of material composition and structure to maximize the benefits of the plasma environment; and investigation of the potential application of a plasma conversion process as part of a CO2 capture cycle. | A1.A8.2 | |
| 18:40 | Authors : C.M. Rangel(1), V.R. Fernandes(1), O. Furtado(1), J. Rodrigues(2) Affiliations : (1) Laboratório Nacional de Energia e Geologia, Estrada do Paço do Lumiar, 22, 1649-038 Lisboa, Portugal, (2) GSyF, Pol. Ind. Alto do Ameal, Pavilhão C-13, 2565-641 Torres Vedras, Portugal Resume : In this work the low temperature electrochemical gasification of graphite in alkaline solutions is explored, taking into account that above the thermodynamic potential for oxygen evolution the faradaic overall current might have a significant contribution from carbon oxidation reactions, seeking the production of synthetic liquid fuels via syngas. Laboratory studies were conducted in an undivided planar cell with graphite electrodes with 25 cm2. Cyclic voltammetry and polarization curves were instrumental to establish optimum operational conditions for adequate molar gases fraction for the production of syngas. Gaseous product analysis was carried out using gas chromatography. A 45W prototype using a 5 cell stack with 150 cm2 was built allowing electrolyte recirculation, temperature control up to 80ºC and pressure control up to 1bar with excellent results. Results and the technological implications of the obtained advances are discussed. KEYWORDS: Hydrogen, Carbon monoxide, Carbon dioxide; Electrochemical gasification; Graphite, Electrolysis. REFERENCES [1]J. Rodrigues, Portuguese Patent 106779 T: Obtenção de gás de síntese por eletrólise alcalina da água, 2013.02.13 | A1.A8.5 | |
No abstract for this day
No abstract for this day
No abstract for this day
No abstract for this day
No abstract for this day
ENSCP / PSL, Paris, France
jacques.amouroux@gmail.com